Check out the Definition, Functions, Classification, Isomerism & Properties of Carbohydrates
Hey, Good to see you here 😀 …… In this Article, We’re gonna discuss about Carbohydrates in Detail….. If you have any queries, don’t forget to mention in Comments….. Thanks
Let’s discuss in brief that What is Carbohydrate?
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates are the polyhydroxy Aldehydes or Ketones, are major macronutrient and the primary sources of energy.
[wp-svg-icons icon=”point-right” wrap=”i”] Consists of Carbon, Hydrogen, and Oxygen.
[wp-svg-icons icon=”point-right” wrap=”i”] The general formula for carbohydrates is Cn(H2O)n.
For e.g. – C6(H2O)6 = C6H12O6
(n=6) (Glucose)
[wp-svg-icons icon=”point-right” wrap=”i”] The sugars which contain Aldehydic group are called as Aldoses & the sugars which contain Ketonic group is called as Ketoses.
What are the functions of Carbohydrates?
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates are the main source of energy.
[wp-svg-icons icon=”point-right” wrap=”i”] 1 gm carbohydrates provide 3.9 Kcal
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates are stored in our body in the form of Glycogen.
[wp-svg-icons icon=”point-right” wrap=”i”] Excess of Carbohydrates converts into fats and stored in Adipose tissues.
[wp-svg-icons icon=”point-right” wrap=”i”] Glycoprotein & Glycolipids are the components of cell membrane and receptors.
[wp-svg-icons icon=”point-right” wrap=”i”] They are the components of Nucleic acids (DNA & RNA).
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates are the structural bases of many organisms. For e.g. – Cellulose in plants & exoskeleton of insects.
[wp-svg-icons icon=”point-right” wrap=”i”] The recommended dietary allowance for carbohydrates is 400gm/ day.
What is the Classification Of Carbohydrates?
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates have been broadly classified into three groups –
1.) Monosaccharides– They are the simple sugars consists of 3 – 7 carbon atoms.
[table id=14 /]
2.) Oligosaccharides– These are the compound sugars which consist of the 2–10 monosaccharides. If an oligosaccharide consists of two monosaccharides, it is called as disaccharides & the one which consists of three monosaccharides, called Trisaccharides & so on….
[table id=15 /]
[table id=16 /]
[table id=17 /]
3.) Polysaccharides– These are the compound sugars which consist of more than 10 molecules of monosaccharides. If polysaccharides consist of the same type of monosaccharides, it is called as Homopolysaccharides & if the polysaccharide consists of different monosaccharides they are called as Heteropolysaccharides.
[table id=18 /]
Isomerism In Carbohydrates
[wp-svg-icons icon=”point-right” wrap=”i”] Isomers are the compounds having same molecular formula but different structural formula and this phenomenon are known as isomerism.
[wp-svg-icons icon=”point-right” wrap=”i”] Isomers are of two types –
- Structural isomers– The isomers having same molecular formula but different structure are called as structural isomers.
- Stereoisomers– The compounds having same molecular formula and structure but differs only in the spatial configuration.
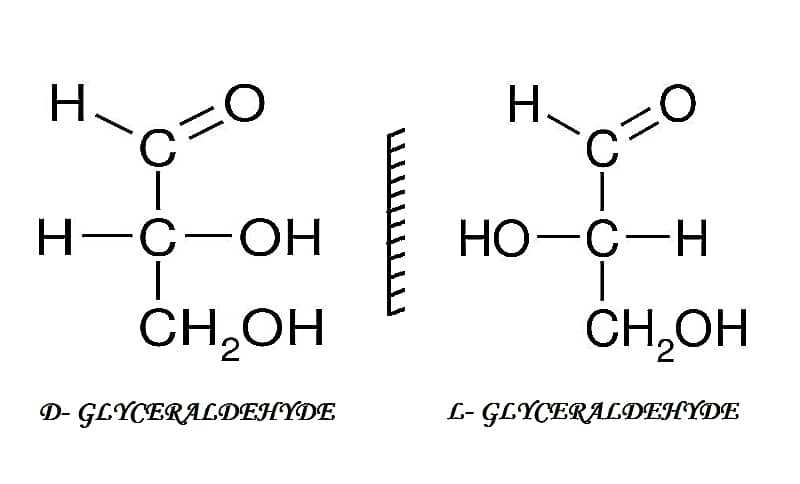
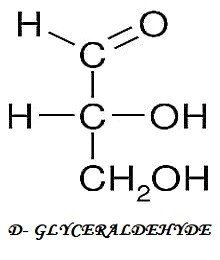
[wp-svg-icons icon=”point-right” wrap=”i”] Enantiomers – They are non-superimposed mirror images of each other. D- and L- isomers are mirror images of each other. When the hydroxyl group is present on the right side the sugar is D- isomer and if it is on the left side it is L- isomer. For e.g. D-Glyceraldehyde and L- Glyceraldehyde.
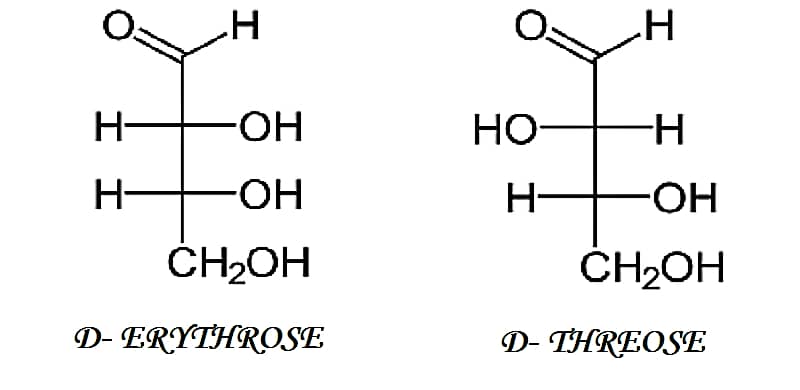
[wp-svg-icons icon=”point-right” wrap=”i”] Diastereomers – The stereoisomers having the different configuration at two or more stereocenters are not the mirror images of each other they are called as diastereomers.
[wp-svg-icons icon=”point-right” wrap=”i”] Optical activity – The substances which rotate the plane polarised light either towards right or towards left are said to be optically active substances and the property is known as optical activity. Those compounds which rotate light towards the right are called as Dextro-rotatory and the compounds which rotate plane polarised light towards left are known as Laevo-rotatory.
Monosaccharide Structures – Fischer Projection Formula Or Open Chain Structures
1.) ALDOSES
I.) 3-C COMPOUND
II.) 4-C COMPOUND
[table id=19 /]
III.) 5-C COMPOUNDS
[table id=20 /]
IV.) 6-C COMPOUND
[table id=21 /]
Here is the Chemical Properties Of Carbohydrates
I.) Tautomerization or Enolisation
[wp-svg-icons icon=”point-right” wrap=”i”] Tautomerization or Enolization is also known as Lobry De Bruyn Alberta’ van ekenstein transformation.
[wp-svg-icons icon=”point-right” wrap=”i”] It is a type of isomerization in which glucose Fructose and mannose are interconvertible in weak alkaline solutions such as sodium hydroxide solution at low temperature.
II.)Reducing property of carbohydrates
[wp-svg-icons icon=”point-right” wrap=”i”] Carbohydrates maybe reducing or nonreducing depending on reducing property of Carbohydrates because of free aldehyde or Ketone group of anomeric carbon.
[wp-svg-icons icon=”point-right” wrap=”i”] Metal hydroxides like Copper Hydroxide oxidized the free aldehyde or Ketone group of reducing sugar.
III.)Oxidation reaction
[wp-svg-icons icon=”point-right” wrap=”i”] The Terminal aldehyde group order terminal alcohol may be oxidized in the presence of the oxidizing agent. For example oxidation of aldehyde group results in the formation of gluconic acid and the oxidation of terminal hydroxyl group results in the formation of glucuronic acid.
IV.)Reduction reaction
[wp-svg-icons icon=”point-right” wrap=”i”] The aldehyde or carbonyl group of monosaccharides are reduced to their corresponding alcohols by treating them with reducing agents like sodium amalgam.
V.)Dehydration reaction
[wp-svg-icons icon=”point-right” wrap=”i”] when carbohydrates treated with concentrated sulphuric acid they undergo dehydration with the elimination of 3 water molecules hexoses gives hydroxymethylfurfural while pentoses give furfurals on dehydration
[wp-svg-icons icon=”point-right” wrap=”i”] The furfural can be condensed with phenolic compounds like Alpha naphthol to form color product ( purple ring) this is the chemical basis of molisch test.
VI.)Osazone (crystal) formation
[wp-svg-icons icon=”point-right” wrap=”i”] When monosaccharides react with phenylhydrazine then it results in the formation of osazones.
[wp-svg-icons icon=”point-right” wrap=”i”] Glucose, Fructose, and mannose give the needle-shaped osazones.
[wp-svg-icons icon=”point-right” wrap=”i”] Maltose forms sunflower type crystals and Lactose forms cotton balls shaped osazones.
VII.)Formation of esters
[wp-svg-icons icon=”point-right” wrap=”i”] The alcoholic group of monosaccharides maybe esterified esterification of Carbohydrates with phosphoric acid is a common reaction in carbohydrates metabolism, for example, Glucose-6-phosphate & Glucose-1-phosphate is formed by esterification of glucose.
VIII.)Mutarotation
[wp-svg-icons icon=”point-right” wrap=”i”] In aqueous solution, the α- & β- anomers of monosaccharides convert into each other by a process called muta-rotation.
IX.)Glycoside formation
[wp-svg-icons icon=”point-right” wrap=”i”] They are formed when hydroxyl group of an anomeric carbon of Carbohydrates reacts with the hydroxyl group of another carbohydrate, the Bond so formed Alpha 1→4 or Beta 1→4 is known as glycosidic Bond.
Frequently Asked Questions (FAQs)
The two main types of isomerism in carbohydrates are stereoisomerism and structural isomerism. Stereoisomerism can be further classified into two subtypes: enantiomers and diastereomers.
Carbohydrates are important sources of energy for the body, and they also play roles in cell communication, cellular structure, and immune system function. Properties of carbohydrates include their solubility in water, tendency to form hydrogen bonds, and their ability to exist as linear or ring-shaped molecules.
The seven functions of carbohydrates are: providing energy, acting as a structural component of cells, regulating blood glucose levels, assisting in protein and lipid metabolism, serving as a signaling molecule in cellular communication, aiding in digestion, and supporting immune system function.
The three main classifications of carbohydrates are monosaccharides, disaccharides, and polysaccharides.
The four main functions of carbohydrates are: providing energy, serving as a structural component of cells, aiding in digestion, and regulating blood glucose levels.
The six main functions of carbohydrates are: providing energy, acting as a structural component of cells, regulating blood glucose levels, assisting in protein and lipid metabolism, serving as a signaling molecule in cellular communication, and supporting immune system function.